Coral Byrns, OMS-IV
VCOM-Auburn
Abstract
This case is to serve as a refresher on methemoglobinemia and the different pathologies, both well understood and newly discovered, that surround it. Patient X, a 66-year-old African American female, was seen in the emergency department with complaints of dyspnea and cough with known COVID-19. She was admitted to the hospital due to hypoxia with COVID-19 pneumonitis. The patient also was noted to have hemolytic anemia at the time of admission. Known comorbid conditions include obesity, erythema dyschromium perstans, ocular sarcoidosis, hyperlipidemia, and hypertension. Of note, she has dapsone, an oxidative drug, on her home medication list. On the first and second day of her hospital stay her oxygen saturation remained low while on BiPAP and, on day three, she was transferred to the intensive care unit. The patient did not receive dapsone inpatient until being transferred to the intensive care unit (day three). The patient’s methemoglobin was 2.7% on day two of admission and went up to 6.4% one day three of admission after receiving dapsone. Dapsone was then discontinued and G6PD levels were ordered. G6PD level testing returned within normal limits. After consulting with the patient’s rheumatologist, it was found she was lost to follow up and had not been on dapsone for several months. While this is likely the reason for her critical methemoglobin levels, it is important to note the elevated methemoglobin on day two of the patient’s hospitalization as well. It has been documented that COVID-19 has shown G6PD levels within normal limits during hospitalization and low levels suggesting deficiency when retested at follow-up. The patient’s methemoglobin trended downwards and was <1% the entirety of her stay. Follow up G6PD levels would have been recommended, but unfortunately the patient passed in the intensive care unit due to many complications regarding her pulmonary status.
Patient X is a 66-year-old African American female who presented to the emergency department with chief complaints of dyspnea and cough. She was brought in by emergency medical services with an Sp02 of 60% on room air. The patient stated that she was seen in a different emergency department five days prior and was diagnosed with COVID-19. She was then discharged home on steroids. This patient had many comorbidities including: morbid obesity (BMI of 53.73), ocular sarcoidosis without known lung involvement, hyperlipidemia, hypertension, and erythema dyschromium perstans. The patient’s home medication list includes albuterol, amlodipine, aspirin, atorvastatin, dapsone, hydrochlorothiazide, and prednisone. In the emergency department the patient was diagnosed with acute hypoxic respiratory failure and COVID-19 pneumonitis (Figure 1.). The patient was weaned from ten liters of oxygen with nasal cannula to six liters and remained in the 90% oxygen saturation range.
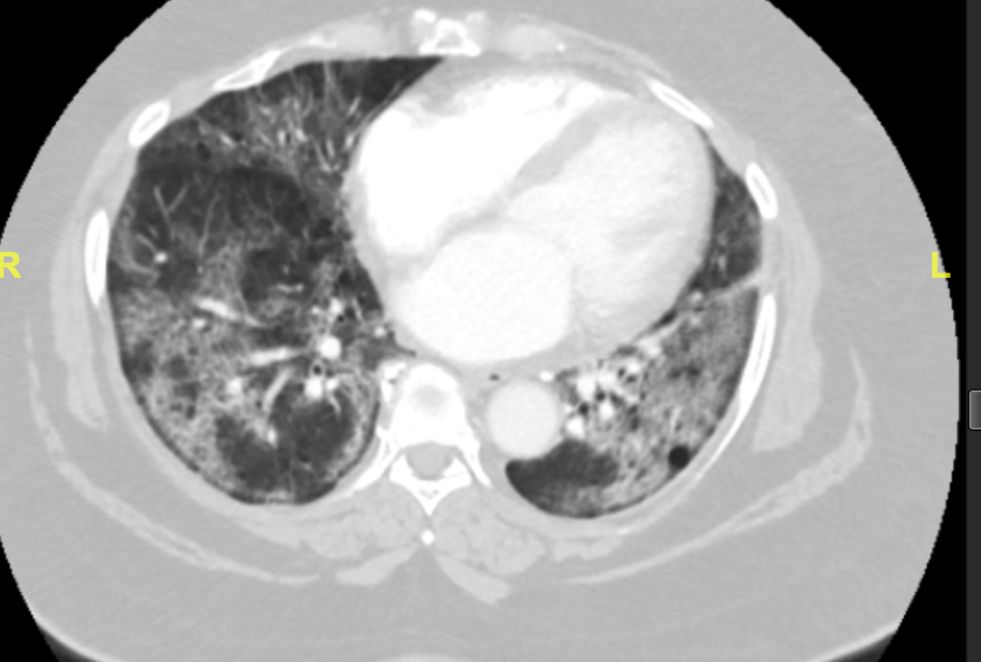
Figure 1. CT showing bilateral diffuse ground glass airspace disease suggesting COVID-19 pneumonitis
The patient was initially admitted to medicine, where her respiratory function continued to decline. She had a trial of BiPAP in the progressive care unit and failed this. During this time, she was treated with remdesivir and dexamethasone for her COVID-19 pneumonitis. She was taking hydroxychloroquine at home for ocular sarcoidosis was discontinued temporarily as it decreases the efficacy of remdesivir. Because she continued to decline, she was intubated, ventilated, and transferred to the intensive care unit on day three.
The initial plan in the ICU included maintenance of mechanical ventilation and finishing her course of remdesivir and dexamethasone. After her course of remdesivir, the plan was to resume her home hydroxychloroquine and dapsone 100 mg, the latter of which had not been administered during her hospitalization yet. Her initial labs in the ICU were significant for respiratory acidosis, hemolytic anemia, methemoglobin of 2.7%, and an elevated white blood cell count of 25,000 meeting SIRS (tachypneic, tachycardic, hypotensive) and sepsis criteria.
The infectious disease (ID) team was consulted at this point in the setting of COVID-19 and sepsis. ID decided to continue dexamethasone/ remdesivir, order blood cultures for sepsis work up, and empirically started the patient on vancomycin and cefepime with plans to de-escalate as needed. ID also mentioned they were unsure why the patient was on dapsone at home and decided to consult the patient’s outpatient rheumatologist. The patient’s rheumatologist noted that the patient takes dapsone for a skin condition called erythema dyschromium perstans. This skin condition is an acquired hyperpigmentation of the dermis and is a variant of lichen planus. The gold standard of treatment is dapsone as it acts as an anti-inflammatory and anti-microbial agent [2.].
On day two in the ICU, the patient continues to have respiratory acidosis. She also had a normocytic hemolytic anemia and her hemoglobin decreased to 7.3, down from 8.5 the day prior. Her white blood cell count improved to 14,000 from 25,000 the day prior. Most critically, the patient had a methemoglobinemia with a methemoglobin of 6.4%, up from 2.7% the day prior. Of note, a normal methemoglobin is <1% unless under oxidative stress [1.] and critical level is anything over 3%. Physiologically speaking, each hemoglobin has a heme group with iron. Usually ferrous (Fe2+) and can bind oxygen and transport it. When an extra electron is lost, Ferrous Fe2+ becomes Ferric Fe3+ which yields methemoglobin. This occurs frequently during oxidative stress. Methemoglobin cannot transport O2 without the lost electron, which yields hypoxemia. Normal methemoglobin is controlled by two mechanisms, major and minor. The minor pathway is a Hexose-monophosphate shunt in RBC (reduced (gain e- back, lose proton) by glutathione). The major shunt is Diaphorase I/II and requires NADH/Cytochrome B5 Reductase/NADPH (reduced by Glucose-6-phosphate dehydrogenase-G6PD and glutathione) [3.,6.].
Oxidative stress is a key factor in methemoglobinemia as the reduction pathways are overwhelmed. In addition to methemoglobinemia, the system is overwhelmed by cellular oxidants caused by infection, a lack of antioxidants, or environmental agents like ozone, drug induced oxidation, or lifestyle choices (smoking, drinking). The reactive oxygen species 02, HOCL, H202, OH) build up during these instances and cannot be reduced fast enough, which yields oxidative stress [1.]. This is known to cause irreversible damage and inflammation to cells/DNA/protein and can be attributed to diseases such as CVD, cancer, Alzheimer’s, COPD, RA, and many others [8.].
Clinically, methemoglobinemia presents as a refractory hypoxemia, which gets worse as the percent of methemoglobin goes up. It is normal to have 100% Sp02 with pulse oximetry with lower PO2 on ABG as pulse oximeters cannot differentiate hemoglobin types. Hemolytic anemia may follow drug induced methemoglobinemia (Heinz bodies/fragmented RBCs) as well. Both of these were seen in our patient. Physical examination differs based on percent of methemoglobin in the blood. With 3-15% (our patient’s category), blue-gray skin can be observed. Higher levels can cause darkening of the blood and can progress to end organ damage and death [6.].
A differential diagnosis for methemoglobinemia includes congenital diseases, such as G6PD deficiency in combination with infection or oxidants (dapsone, fava beans, nitrous oxide, sulfa drugs), Cytochrome B5 Reductase Deficiency, or Hemoglobin M and E variants. Acquired variants (most common) include sepsis (release of NO), exposure to oxidant drugs, chemicals, or toxins (dapsone, benzocaine, nitroglycerin, hydroxychloroquine), and possibly hemolytic crisis. In our patient, we considered G6PD deficiency due to her risk factor of being African American [3.]. We also considered her use of dapsone, though it was our understanding that the patient has been on dapsone for months and her methemoglobin was <1% on initial presentation. Additionally, her methemoglobin level was 2.7% before receiving any dapsone.
With this newfound critical methemoglobinemia in patient X, ID was consulted again. They decided to order G6PD levels and discontinue dapsone as it is a well-known cause of oxidative stress [7.]. The patient’s rheumatologist was consulted once again to determine if the patient had been tested for G6PD in the past with her being on dapsone. At this time, it was discovered that she was lost to follow up after her first prescription of dapsone (where they would test for G6PD deficiency) over three months ago and had not been on the medication since.
On day three in the ICU, one day after critical methemoglobin level and discontinuation of dapsone, the patient’s methemoglobin was down to 1.7. Dapsone has a half-life of 24 hours, so the level was expected to continue to decline to <1%. It was <1% on day four. On this day her G6PD levels came back within normal limits at 5.4 U/g. With her levels down, there was no need for medical treatment. Methylene blue is the gold standard treatment for methemoglobinemia. This cannot be given to patients with G6PD deficiency as G6PD is required for methylene blue to aid in the reduction of ferric iron to ferrous iron. In these patients, hyperbaric oxygen can be considered to oxygenate the tissues peripherally [4.].
Following resolution of methemoglobinemia on ICU Day 3, Patient X’s methemoglobin level continued to remain low. Unfortunately, this patient expired on day 56 of her hospitalization due to multiple comorbidities (both new and old) experienced inpatient. These include acute renal failure, left pneumothorax, COVID-19 pneumonitis, sepsis, sarcoidosis, acute hypoxemic and hypercapnic respiratory failure, hyperglycemia, fluid overload, morbid obesity, thrombocytopenia, atelectasis, and hemolytic anemia.
Interestingly, research has been done on methemoglobinemia and hemolytic anemia after COVID-19. Once specific case study did not involve an eliciting drug. This study found methemoglobinemia (10.5%) in a patient whose G6PD levels were within normal limits while hospitalized (7.7 U/g) (normal 4.5-13.5). This patient was not on oxidizing drugs and had no history of taking any. This patient had COVID-19 along with hemolytic anemia (Hb 7.9 g/dL) with elevated LDH (2882 u/L) and bilirubin (2.23 mg/dL). At a five month follow up retest of G6PD levels, they were low (3.7 U/g) and the patient was diagnosed with G6PD deficiency [5.]. This study serves two important points: be aware of possible false negative G6PD testing during hemolytic crisis and the importance of follow up, as well as the role COVID-19 contributes to oxidative stress.
In conclusion, this case of methemoglobinemia was likely due to the patient’s one-time 100 mg dose of dapsone given in the hospital. Consideration for follow up is always recommended in these patients post-hospitalization and primary care physicians should consider serial monitoring G6PD levels in this type of patient. Our patient had a slight bump in methemoglobin before dapsone was given. This could have been related to her possible COVID-19 related hemolysis, so rechecking her G6PD outpatient would have been recommended to confirm the levels were not falsely elevated in the hospital. Overall, methemoglobinemia can be covered up by several comorbidities, especially when oxidative stress could be coming from more than one predisposing factor. This should be kept in mind when obtaining family histories, medication lists, past medical history/ family history, and physical examination/physical exam.
Resources
[1.]. Chatterjee, S. (2016, June 3). Oxidative stress, inflammation, and disease. Oxidative Stress and Biomaterials. Retrieved March 28, 2022, from https://www.sciencedirect.com/science/article/pii/B9780128032695000024
[2.]. Erythema dyschromicum perstans. Erythema dyschromicum perstans (ashy dermatosis) | DermNet NZ. (n.d.). Retrieved March 28, 2022, from https://dermnetnz.org/topics/erythema-dyschromicum-perstans
[3.]. G6PD Deficiency . Cedars Sinai. (n.d.). Retrieved March 28, 2022, from https://www.cedars-sinai.org/health-library/diseases-and-conditions/g/g6pd-deficiency.html
[4.]. Khaled F Abouelezz, M. B. C. B. (2021, November 5). Methemoglobinemia Treatment & Management: Approach considerations, initial management, pharmacologic therapy, exchange transfusion, and hyperbaric oxygen. Methemoglobinemia Treatment & Management: Approach Considerations, Initial Management, Pharmacologic Therapy, Exchange Transfusion, and Hyperbaric Oxygen. Retrieved March 28, 2022, from https://emedicine.medscape.com/article/204178-treatment#d8
[5.]. Lopes, D. V., Neto, F. L., Marques, L. C., Lima, R. B. O., & Brandão, A. A. G. S. (2020, November 19). Methemoglobinemia and hemolytic anemia after COVID-19 infection without identifiable eliciting drug: A case-report. IDCases. Retrieved March 28, 2022, from https://www.sciencedirect.com/science/article/pii/S2214250920303218#bib0045
[6.]. Methemoglobinemia. WikEM. (n.d.). Retrieved March 28, 2022, from https://wikem.org/wiki/Methemoglobinemia#Differential_Diagnosis
[7.]. Olteanu, H., Harrington, A. M., George, B., Hari, P. N., Bredeson, C., & Kroft, S. H. (2011, April 11). High prevalence of dapsone-induced oxidant hemolysis in North American SCT recipients without glucose-6-phosphate-dehydrogenase deficiency. Nature News. Retrieved March 28, 2022, from https://www.nature.com/articles/bmt201183
[8.] Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D., & Bitto, A. (2017). Oxidative stress: Harms and benefits for human health. Oxidative medicine and cellular longevity. Retrieved March 28, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5551541/
