Author: Thomas Mathis MS-3, AEMT, USAR MC
Campbell University School of Osteopathic Medicine
Introduction
Damage Control Resuscitation (DCR) is a methodology for stabilizing severely injured trauma patients with massive hemorrhage by aggressively pursuing hemostasis and proactively addressing the lethal triad of coagulopathy, acidosis, and hypothermia.1,2,3 Developed over the last fifty years, DCR employs such strategies as rapid hemorrhage control, permissive hypotension, blood product transfusion, and active rewarming to prolong patient survival and provide stabilization prior to definitive treatment.2,3 These modalities have spurred the development of new medical practices and products, which have influenced clinical decision making at all points along the continuum of care, from the prehospital system to the operating room. This review of current literature hopes to illustrate the clinical reasoning behind the practice of Damage Control Resuscitation, depict its application and benefit for emergency medicine providers, and share new innovations in the care of trauma patients which will shape the future direction of DCR.
Pathophysiology
Hemorrhagic shock is the primary physiological insult addressed by DCR. Massive blood loss due to injury results in rapid undermining of the clotting process, known as Trauma Induced Coagulopathy (TIC). Roughly 30% of all trauma patients will present with a biochemically evident TIC upon arrival in the emergency department.3,4 TIC is a nuanced process with a diversity of phenotypic expression. Coagulopathy from trauma stems from both hyperfibrinolysis and hypofibrinolysis, endothelial dysfunction, and platelet derangement.5
Following traumatic insult, tissue injury and hypoperfusion drive the vascular system to increase blood flow. The resulting sympathetic surge is followed by overexpression of thrombomodulin by the endothelium, which binds to thrombin and Endothelial Protein C Receptor (EPCR) in order to activate Protein C (aPC). aPC is a fibrinolytic that inactivates Factors Va and VIIIa as well as Plasminogen Activator Inhibitor 1 (PAI-1).5,6 The resultant hypocoagulation increases blood flow but exacerbates exsanguination and is the principal pathway in early TIC. Concurrently, damage to the vascular endothelium causes release of endogenous heparin sulfate into the bloodstream, contributing to further anticoagulation.7 After 6-24 hours, the mechanisms of late TIC take effect, transitioning the patient from hypocoagulation to a hypercoagulable state.6 Among other factors, this transition is precipitated by endothelial injury, platelet activation and exhaustion of Protein C, which leads to an increase in both macro and micro-clotting through unchecked thrombin activation.4,6 Subsequent venous thromboembolism and multiple organ failure are common.6 While early and late TIC are presented as distinct dysfunctions, the characteristics of both often exist together in a mixed presentation that is nuanced in origin and patient dependent.6,7
Trauma Induced Coagulopathy is only one vertex in the lethal triad of hemorrhagic shock that is addressed by DCR; it is accompanied by acidosis and hypothermia.4 These dysfunctions can exacerbate TIC while creating distinct pathology of their own. Hypothermia, caused by cold fluid infusion and patient exposure, has been shown to decrease the synthesis of fibrinogen while negatively impacting platelet function.4 Additionally, it is thought to slow the reaction velocity of key biochemical reactions along the coagulation cascade. Hypothermia in trauma is associated with increased transfusion requirements and elevated INR as well as increased ICU and ventilator days.8 Acidosis, stemming from hypoperfusion and the subsequent generation of lactate, denatures clotting factors, decreases thrombin synthesis, and nearly completely inhibits ADP-mediated platelet aggregation.4 Additionally in recent years, hypocalcemia has gained increasing attention as an aggravating factor of the lethal triad. Up to 55% of all trauma patients display hypocalcemia prior to transfusion, and these findings are only worsened by citrate found in blood products. This problem is so commonplace that some have called for its inclusion in the “lethal diamond,” and the US military recommends one gram of calcium be administered with every four units of blood products.9 The synergic effects of hypocalcemia, with TIC, acidosis, and hypothermia lead to significant morbidity and mortality that is hard to reverse once established. DCR was developed to preempt this self-propagating cycle of hemorrhagic shock before it becomes lethal.
Fundamental Principles
Damage Control Resuscitation is an umbrella term for actions directed toward early and rapid hemostasis, thus preventing TIC and the remainder of the lethal triad from propagating. Fundamentally, these actions include expedient hemorrhage control, 1:1:1 blood product transfusion, permissive hypotension, and prevention of hypothermia.2,3 These interventions interface with the actions taken during Damage Control Surgery (DCS), whereby abbreviated repair of traumatic injury is undertaken to mitigate blood loss and wound contamination. The end state achieved by DCR/DCS is intensive physiological restoration with staged anatomical reconstruction only once the patient is stable enough to tolerate additional surgery.10,11,12
Early hemorrhage control is the cornerstone of DCR and the first intervention that should be attempted for actively bleeding trauma patients. In one prospective, randomized observational study of hemorrhaging patients, 94% of mortality occurred within three hours of initial injury.13 Without arresting hemorrhage, it is nearly impossible to return the patient to a normal physiological state, and exsanguination is likely.14 For extremities and areas of juncture, commercially produced compressive dressings, hemostatic gauze, extremity/junctional tourniquets, and pelvic binders should be used.15 A retrospective, multicenter analysis of peripheral vascular injuries conducted in 2018 found that tourniquet use was associated with a significant increase in patient survival and that risk of amputation was not significantly different.16 However, isolated extremity injury is exceedingly rare in civilian trauma, with one review of a New York state trauma registry showing peripheral vascular injury in only 1.8% of patients. In a review of public mass shooting fatalities, 51% of deaths were attributed to thoracic wounds while 39% were from head injuries.17 Clearly, additional methods of bleeding control must be adopted for the types of hemorrhage seen in the majority of trauma victims.
To this end, tranexamic acid (TXA) has seen a rise in utilization. By blocking the lysine-binding site of plasminogen, TXA prevents plasmin activation and fibrinolysis, halting clot degradation and stemming the deleterious effects of early TIC.18 The CRASH-2 study, a groundbreaking randomized controlled trial from 2013, found that TXA reduced mortality in trauma patients by 15% when given within three hours of injury.19 While further study is still needed, TXA has been found safe to use in traumatic brain injury and does not appear to increase thrombotic events in trauma surgery patients, although it should be avoided in patients with prothrombotic states.20 The future potential of TXA promises a reliable method for hemorrhage control for internal injuries that cannot be addressed with conventional tourniquets or compressive dressings. However, all of these methods are adjunctive and cannot replace prompt surgical intervention as the gold standard in arresting hemorrhage.
Timely blood product transfusion is an equally important step in establishing hemostasis and hemodynamic stability for trauma patients during DCR. Packed Red Blood Cells (pRBCs) provide oxygen carrying capacity to combat ischemia and acidosis, while platelets and plasma replace clotting factors in order to mitigate early TIC.21 While it is tempting to temporize patient physiology with crystalloid infusion, this has repeatedly been shown to drastically increase mortality.22,23,24 Current practice in the hospital setting is to transfuse as close to a 1:1:1 ratio of warmed pRBCs, plasma, and platelets in patients who meet massive transfusion protocol (MTP).21 This strategy attempts to reconstitute the lost whole blood for which the components are replacing. In 2014, the PROMMTT study demonstrated through a randomized controlled trial that an early transfusion strategy high in plasma and platelets led to a profound decrease in mortality in the first six hours of treatment.13 These findings were followed by the PROPPR study, which found that there was no difference in survival or adverse effects between patients receiving a high ratio or low ratio of platelets, 1:1:1 or 1:1:2 respectively. However, the 1:1:1 group saw a significant decrease in mortality from exsanguination in the first six hours of injury.25 These recent studies cement transfusion as an essential practice in DCR, and current efforts are being made to extend its benefits to the prehospital environment as well.
Permissive hypotension and hypothermia prevention constitute the remaining fundamental principles of DCR. In cases of non-compressible hemorrhage, clotting at the site may be the only impediment to exsanguination. In 1994, Bickel et al. demonstrated increased survival in trauma patients who received no crystalloid fluids for perfusion support in the prehospital setting.22 This was followed in 2003 by Sondeen et al., who determined that rebleeding at swine aortic lesions occurred with a mean arterial pressure (MAP) of 64 mmHg and a systolic blood pressure (SBP) of 94 mmHg, regardless of lesion size.26 Today, the standard practice of permissive hypotension is to maintain a SBP of 80-90 mmHg, or a MAP of >80 mmHg in head trauma, until surgical hemorrhage control is achieved.18,27,28 Additionally, hypothermia can lead to significant morbidity and mortality if not addressed.8 A recent analysis of the Department of Defense Trauma Registry found that a temperature as high as 97.1oF was associated with increased mortality and that 14% of the patients studied had temperatures below this value.29 External warming methods act to prevent heat loss but are ineffective in transferring heat internally to the patient. As such, warmed blood product transfusion and early hemorrhage control in addition to heated blankets are the most practical means of preventing hypothermia in the emergency setting.8
New Innovations
While DCR has matured in recent decades, there are still gaps in capability and understanding that, if corrected, promise further reductions in morbidity and mortality. Among these are improved transfusion practices in both the prehospital and hospital setting, better laboratory monitoring of coagulation in trauma patients, and non-surgical hemorrhage control of massive bleeding. A growing body of literature has reinforced these potential advances and many groundbreaking organizations have seen positive results with their adoption.
A transfusion strategy able to provide plasma and RBCs in a rapid manner is the preferred method in DCR, and many trauma systems have adopted Whole Blood (WB) as a resuscitation fluid accordingly. WB delivers all elements of component therapy in a single product, simplifying logistics and expediting initiation of transfusion. In 2014, the US Military’s Committee on Tactical Combat Casualty Care (CoTCCC) recommended WB as the fluid of choice for combat casualties.30 Since 2018, the Southwest Texas Regional Advisory Council trauma service has performed WB prehospital transfusions in both urban and rural environments by air and ground transport. In retrospective analysis, prehospital WB transfusions showed decreased incidence of massive transfusion events compared to no prehospital transfusion. Additionally, patients receiving prehospital WB required less initial volume replacement. ED mortality was reduced and length of stay mortality trended toward improvement.31 A critical review of low titer O-negative WB usage in civilian hospital trauma care revealed promising absolute mortality benefit compared to component therapy in seven trials, but these were retrospective analyses and struggled to minimize confounds.32 Compelling randomized control trials for WB in DCR are ongoing.
Whole blood transfusion excluded, Damage Control Resuscitation still relies on empiric transfusion of blood products in a 1:1:1 fixed ratio of pRBCs, plasma, and platelets. Conventional coagulation studies are slow to conduct and often do not reflect the hematological status of trauma patients accurately, limiting their utility in tailoring resuscitation to individual patients. However, new advances in rapid, viscoelastic testing and impedance aggregometry have allowed for point-of-care, clinical monitoring of clot initiation, progression, strength, and lysis, enabling goal directed transfusion in DCR.33 Initially developed for liver transplant surgery, commercially available forms of viscoelastic testing include thromboelastography (TEG) and rotational thromboelastometry (ROTEM). Rapid thromboelastography (rTEG) can yield preliminary coagulation data in as little as five minutes. TEG and ROTEM guided resuscitation has been shown to improve mortality compared with fixed ratio, component therapy in multiple retrospective, observational studies.34 In 2016, a prospective, randomized control trial found significantly decreased mortality for trauma patients at six hours and 28 days when TEG-guided DCR was employed versus conventional coagulation studies.35 Additionally, viscoelastic testing may increase the safety of TXA use, as early coagulation profiling may identify hypofibrinolysis in trauma patients before it is administered.33 More high-quality trials are still needed, but point-of-care coagulation testing portends the maturation of goal directed DCR and the possible antiquation of fixed ratio transfusion in the hospital setting.
Despite improvements to transfusion practices, noncompressible hemorrhage control remains a difficult challenge for DCR, especially in the critical period before surgical control of bleeding has been achieved. While pelvic binders, wound packing, and bone fracture splinting are reasonable adjuncts for internal hemorrhage, these provide very little capability to monitor and influence hemodynamic stability. To this end, resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a tantalizing procedure to temporize critically exsanguinating trauma victims in the prehospital, emergency department, or operating room settings.37 By inflating a balloon in the descending thoracic aorta or in the abdominal aorta below the renal arteries, severe bleeding can be stopped or limited in thoracic, abdominal, pelvic, or junctional injuries.38 While its adverse effects include acute renal injury, ischemia-reperfusion syndrome, and amputation, the advantages of REBOA could be pivotal to a select subset of trauma patients with refractory hypovolemic shock secondary to subdiaphragmatic hemorrhage, providing real time blood pressure monitoring and added time to deliver the patient to competent surgical care before exsanguination.39,40 Many trauma systems have adopted this procedure as a routine practice. The United Kingdom’s Defense Medical Services now offer REBOA at all deployed role two medical units.41 The Trauma and Emergency Surgery Group of Cali, Columbia utilizes REBOA in all blunt and penetrating trauma patients with a refractory SBP of less than 70 mmHg.42 Preliminary data from France, The United States, and Great Britain have illustrated potential morbidity and mortality benefit with REBOA utilized by hospital-based and retrieval specialists.43,44,45,46 However, retrospective analysis of patients with pelvic fracture, blunt trauma, or hemodynamic instability who received REBOA found that MAP significantly increased as a result of REBOA placement but that absolute mortality was still less than surgical cavity packing.47 Still in its infancy, REBOA has a myriad of potential advantages and applications with promising initial evidence of its utility, but it is hampered by a lack of prospective study.
DCR for the Prehospital System, Emergency Department, and Operating Room / ICU
Figure 1. contains the most recent developments in DCR care at all stages along the chain of survival. While not a replacement for a comprehensive trauma protocol, such as advanced trauma life support (ATLS), it does provide indications for the initiation of DCR and the critical steps necessary to achieve its goals, namely hemostasis and preemptive management of the lethal triad. While many of the action items are not yet considered standard of care for trauma management, they represent a future vision for DCR based on ongoing innovations and a review of current literature.
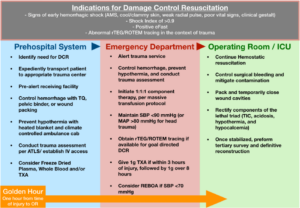
Figure 1. demonstrates the functions of a well orchestrated damage control resuscitation system at all levels of its use. Based on current trends and potential innovations, this infographic is not intended to replace any peer-reviewed protocols, but instead acts as a representation of DCRs future potential in a robust trauma system.
Conclusion
Damage control resuscitation is an effective methodology for achieving early hemostasis and decreasing mortality in hemorrhagic shock and trauma induced coagulopathy. DCR’s fundamental techniques of hemorrhage control, permissive hypotension, blood product transfusion, and hypothermia prevention have been validated by prospective study and are currently in use in trauma systems throughout the world. Advances such as whole blood transfusion, point-of-care testing, and REBOA promise to improve upon DCR’s successes and expand its capabilities in the Emergency Department and prehospital setting. With the efforts of progressive organizations, such as the military medical system and intensive civilian trauma centers, damage control resuscitation can be matured, and more lives can be saved from injuries once thought to be irredeemable.
References
- Samuels JM, Moore HB, Moore EE. Damage Control Resuscitation. Chirurgia (Bucur). 2017;112(5):514-523. doi:10.21614/chirurgia.112.5.514
- Bogert JN, Harvin JA, Cotton BA. Damage Control Resuscitation. J Intensive Care Med. 2016;31(3):177-186. doi:10.1177/0885066614558018
- Andreason CL, Pohlman TH. Damage Control Resuscitation for Catastrophic Bleeding. Oral Maxillofac Surg Clin North Am. 2016;28(4):553-568. doi:10.1016/j.coms.2016.06.010
- Fecher A, Stimpson A, Ferrigno L, Pohlman TH. The Pathophysiology and Management of Hemorrhagic Shock in the Polytrauma Patient. J Clin Med. 2021;10(20):4793. Published 2021 Oct 19. doi:10.3390/jcm10204793
- Ho VK, Wong J, Martinez A, Winearls J. Trauma-induced coagulopathy: Mechanisms and clinical management. Ann Acad Med Singap. 2022;51(1):40-48. doi:10.47102/annals-acadmedsg.2020381
- Duque P, Calvo A, Lockie C, Schöchl H. Pathophysiology of Trauma-Induced Coagulopathy. Transfus Med Rev. 2021;35(4):80-86. doi:10.1016/j.tmrv.2021.07.004
- Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: The past, present, and future. J Thromb Haemost. 2019;17(6):852-862. doi:10.1111/jth.14450
- van Veelen MJ, Brodmann Maeder M. Hypothermia in Trauma. Int J Environ Res Public Health. 2021;18(16):8719. Published 2021 Aug 18. doi:10.3390/ijerph18168719
- Ditzel RM Jr, Anderson JL, Eisenhart WJ, et al. A review of transfusion- and trauma-induced hypocalcemia: Is it time to change the lethal triad to the lethal diamond?. J Trauma Acute Care Surg. 2020;88(3):434-439. doi:10.1097/TA.0000000000002570
- Tien H, Beckett A, Garraway N, Talbot M, Pannell D, Alabbasi T. Advances in damage control resuscitation and surgery: implications on the organization of future military field forces. Can J Surg. 2015;58(3 Suppl 3):S91-S97. doi:10.1503/cjs.001815
- Benz D, Balogh ZJ. Damage control surgery: current state and future directions. Curr Opin Crit Care. 2017;23(6):491-497. doi:10.1097/MCC.0000000000000465
- Ordoñez CA, Parra MW, Serna JJ, et al. Damage control resuscitation: REBOA as the new fourth pillar. Colomb Med (Cali). 2020;51(4):e4014353. Published 2020 Dec 30. doi:10.25100/cm.v51i4.4353
- Holcomb JB, del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127-136. doi:10.1001/2013.jamasurg.387
- Meléndez-Lugo JJ, Caicedo Y, Guzmán-Rodríguez M, et al. Prehospital Damage Control: The Management of Volume, Temperature… and Bleeding!. Colomb Med (Cali). 2020;51(4):e4024486. Published 2020 Dec 30. doi:10.25100/cm.v51i4.4486
- Kao RL. Introduction: combat damage-control resuscitation/surgery and beyond. Can J Surg. 2018;61(6):S178-S179. doi:10.1503/cjs.014418
- Teixeira PGR, Brown CVR, Emigh B, et al. Civilian Prehospital Tourniquet Use Is Associated with Improved Survival in Patients with Peripheral Vascular Injury. J Am Coll Surg. 2018;226(5):769-776.e1. doi:10.1016/j.jamcollsurg.2018.01.047
- Hsu YT, Chang DC, Perez NP, et al. Civilian Firearm-related Injuries: How Often is a Tourniquet Beneficial?. Ann Surg. 2020;271(2):e12-e13. doi:10.1097/SLA.0000000000003622
- Giannoudi M, Harwood P. Damage control resuscitation: lessons learned. Eur J Trauma Emerg Surg. 2016;42(3):273-282. doi:10.1007/s00068-015-0628-3
- Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1-79. doi:10.3310/hta17100
- Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: An illustrated review. Res Pract Thromb Haemost. 2021;5(5):e12546. Published 2021 Jul 14. doi:10.1002/rth2.12546
- Kalkwarf KJ, Cotton BA. Resuscitation for Hypovolemic Shock. Surg Clin North Am. 2017;97(6):1307-1321. doi:10.1016/j.suc.2017.07.011
- Bickell WH, Wall MJ Jr, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105-1109. doi:10.1056/NEJM199410273311701
- Harris T, Davenport R, Mak M, Brohi K. The Evolving Science of Trauma Resuscitation. Emerg Med Clin North Am. 2018;36(1):85-106. doi:10.1016/j.emc.2017.08.009
- Geeraedts LM Jr, Pothof LA, Caldwell E, de Lange-de Klerk ES, D’Amours SK. Prehospital fluid resuscitation in hypotensive trauma patients: do we need a tailored approach?. Injury. 2015;46(1):4-9. doi:10.1016/j.injury.2014.08.001
- Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-482. doi:10.1001/jama.2015.12
- Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rebleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54(5 Suppl):S110-S117. doi:10.1097/01.TA.0000047220.81795.3D
- Dünser MW, Takala J, Brunauer A, Bakker J. Re-thinking resuscitation: leaving blood pressure cosmetics behind and moving forward to permissive hypotension and a tissue perfusion-based approach. Crit Care. 2013;17(5):326. Published 2013 Oct 8. doi:10.1186/cc12727
- Weingart S, Borshoff D. The Resuscitation Crisis Manual. West Perth, W.A.: Leeuwin Press; 2018.
- Schauer SG, April MD, Fisher AD, et al. Hypothermia in the Combat Trauma Population [published online ahead of print, 2022 Sep 19]. Prehosp Emerg Care. 2022;1-7. doi:10.1080/10903127.2022.2119315
- Clarke EE, Hamm J, Fisher AD, et al. Trends in Prehospital Blood, Crystalloid, and Colloid Administration in Accordance With Changes in Tactical Combat Casualty Care Guidelines [published online ahead of print, 2021 Dec 22]. Mil Med. 2021;usab461. doi:10.1093/milmed/usab461
- Braverman MA, Smith A, Pokorny D, Axtman B, Shahan CP, Barry L, Corral H, Jonas RB, Shiels M, Schaefer R, Epley E, Winckler C, Waltman E, Eastridge BJ, Nicholson SE, Stewart RM, Jenkins DH. Prehospital whole blood reduces early mortality in patients with hemorrhagic shock. Transfusion. 2021 Jul;61 Suppl 1:S15-S21. doi: 10.1111/trf.16528. PMID: 34269467.
- Jackson B, Murphy C, Fontaine MJ. Current state of whole blood transfusion for civilian trauma resuscitation. Transfusion. 2020;60 Suppl 3:S45-S52. doi:10.1111/trf.15703
- Hanke AA, Horstmann H, Wilhelmi M. Point-of-care monitoring for the management of trauma-induced bleeding. Curr Opin Anaesthesiol. 2017;30(2):250-256. doi:10.1097/ACO.0000000000000448
- Brill JB, Brenner M, Duchesne J, et al. The Role of TEG and ROTEM in Damage Control Resuscitation. Shock. 2021;56(1S):52-61. doi:10.1097/SHK.0000000000001686
- Gonzalez E, Moore EE, Moore HB, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Ann Surg. 2016;263(6):1051-1059. doi:10.1097/SLA.0000000000001608
- Tapia NM, Chang A, Norman M, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378-386. doi:10.1097/TA.0b013e31827e20e0
- Ellis DY. REBOA: Where are we now?. Emerg Med Australas. 2020;32(1):4-6. doi:10.1111/1742-6723.13432
- Marciniuk P, Pawlaczyk R, Rogowski J, Wojciechowski J, Znaniecki Ł. REBOA – new era of bleeding control, literature review. Pol Przegl Chir. 2019;92(2):42-47. doi:10.5604/01.3001.0013.5426
- Ribeiro Junior MAF, Feng CYD, Nguyen ATM, et al. The complications associated with Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). World J Emerg Surg. 2018;13:20. Published 2018 May 11. doi:10.1186/s13017-018-0181-6
- Cantle PM. REBOA utility. Surg Open Sci. 2022;8:50-56. Published 2022 Mar 18. doi:10.1016/j.sopen.2022.03.002
- Marsden MER, Buckley AM, Park C, Tai N, Rees P. Balloons on the battlefield: REBOA implementation in the UK Defence Medical Services [published online ahead of print, 2021 Aug 18]. BMJ Mil Health. 2021;bmjmilitary-2021-001925. doi:10.1136/bmjmilitary-2021-001925
- Ordoñez CA, Parra MW, Serna JJ, et al. Damage control resuscitation: REBOA as the new fourth pillar. Colomb Med (Cali). 2020;51(4):e4014353. Published 2020 Dec 30. doi:10.25100/cm.v51i4.4353
- Ellis DY. REBOA: Where are we now?. Emerg Med Australas. 2020;32(1):4-6. doi:10.1111/1742-6723.13432
- Pieper A, Thony F, Brun J, et al. Resuscitative endovascular balloon occlusion of the aorta for pelvic blunt trauma and life-threatening hemorrhage: A 20-year experience in a Level I trauma center. J Trauma Acute Care Surg. 2018;84(3):449-453. doi:10.1097/TA.0000000000001794
- Brenner M, Teeter W, Hoehn M, et al. Use of Resuscitative Endovascular Balloon Occlusion of the Aorta for Proximal Aortic Control in Patients With Severe Hemorrhage and Arrest. JAMA Surg. 2018;153(2):130-135. doi:10.1001/jamasurg.2017.3549
- Lendrum R, Perkins Z, Chana M, et al. Pre-hospital Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation. 2019;135:6-13. doi:10.1016/j.resuscitation.2018.12.018
- Frassini S, Gupta S, Granieri S, et al. Emergency Management of Pelvic Bleeding. J Clin Med. 2021;10(1):129. Published 2021 Jan 1. doi:10.3390/jcm10010129
