Kara Hatlevoll DO, MS, FAWM*, Harshit Singh*, Jaime Weber MS*, Natalie Griego MD
*These authors contributed equally to this work.
Abstract
Case Presentation: An 82-year old patient with preexisting COVID-19 pneumonia and significant cardiac risk factors presented via EMS to the emergency department for weakness, shortness of breath, and hypoxia at her nursing home. Vital signs on arrival were significant for hypertension, hypoxia, tachypnea, and fever.
Results: Lab work performed in the emergency department showed an elevated troponin and ECG revealed ST elevations in leads II, III, and V6. On cardiac catheterization, the patient was found to have no significant atherosclerotic disease, but her ejection fraction was 25-30%.
Conclusion: In the setting of significant physiologic stress secondary to COVID-19 pneumonia, this patient likely suffered from Takotsubo cardiomyopathy. Her cardiomyopathy was responsible for an elevated troponin and ECG changes that suggested ischemia.
Case
An 82-year old COVID-positive female with a history of hypertension, dementia, anxiety, and hyperlipidemia arrived at the emergency department with weakness and “shortness of air.” The patient was a poor historian, and the history was provided by EMS and the patient’s family. The patient’s family stated that she had been admitted to a COVID unit in her nursing home, where her oxygen saturation dropped to 85% on room air. Shortly following this decline, the family requested her transfer to the emergency department for further evaluation. Upon arrival, the patient appeared to have an altered level of consciousness. Vital signs were significant for a SpO2 of 92% on 2L by nasal cannula, blood pressure of 167/69, and a fever of 38.1 C. On physical exam, the patient was noted to be somnolent. Breath sounds were diminished bilaterally but with no wheezes, rales, or rhonchi. The patient did not appear to be in respiratory distress. No new murmurs or rubs were noted on the cardiovascular exam, and the rest of her exam was otherwise unremarkable with no signs of heart failure.
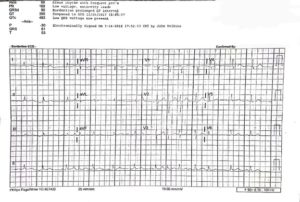
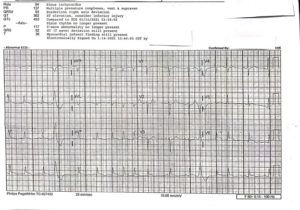
On arrival, the initial ECG (Figure 1) showed no definitive STEMI, although subtle ischemic changes were present along with a mildly prolonged QTC interval. Laboratory evaluation was significant for an elevated troponin of 3.23 ng/mL (NL < 0.06 ng/mL). Aspirin, heparin, and IV fluids were given. Chest X-Ray revealed atelectasis and focal consolidation suspicious for pneumonia in the right costophrenic angle with diffuse increased interstitial lung markings consistent with COVID pneumonia. Two hours later, a repeat ECG showed new ST elevations in leads II, III, and V6 (Figure 2). Cardiac catheterization showed no significant coronary artery disease, but ejection fraction was found to be 25-30%, with wall motion abnormalities present, suggesting non-ischemic cardiomyopathy.
The patient was admitted to the hospital and started on IV hydration, IV steroids, and antibiotics for potential secondary bacterial pneumonia. The patient’s status later decompensated, and she was admitted to the ICU and started on Remdesivir and Lovenox. Unfortunately, these treatments were not successful, and the patient’s respiratory failure eventually led to her death.
Figure 1: Initial ECG. (Click illustration to enlarge)
Figure 2: ECG showing signs of ischemia. (Click illustration to enlarge)
Discussion
Takotsubo cardiomyopathy is a form of non-ischemic cardiomyopathy, presenting as symptomatic left ventricular systolic dysfunction and heart failure in the absence of underlying coronary artery disease. Clinically, patients present with chest pain, dyspnea, and ECG findings consistent with STEMI (1). This patient’s presentation of chest pain, elevated troponin, and ECG with apparent STEMI and confirmed wall motion abnormalities, but a clean catheterization is characteristic of Takotsubo cardiomyopathy.
Also known as broken heart syndrome, Takotsubo cardiomyopathy is more commonly found in women, approximately 9:1 compared to men, and can be triggered by emotional and physiological stressors, such as infections (8,9). It has been proposed that increased sympathetic activity, catecholamine release, and microvascular spasms result in left ventricular dysfunction. The resultant dysfunction can include a distribution beyond that of a specific coronary artery, producing ST-segment elevation and wall motion abnormality. Notably, COVID-19 has been shown to cause significant multi-organ stress on the body, and cases of various types of cardiomyopathy have been documented. These cases range from post-viral myocarditis to Takotsubo cardiomyopathy (3). As we see in the literature, risk factors for the development of cardiomyopathy following COVID-19 infections include: age greater than 55, obesity, and elevated troponin (4), all of which our patient presented with.
This patient’s cardiac risk factors of hypertension and hyperlipidemia, in the setting of severe COVID-19 infection with the presence of non-ischemic cardiomyopathy, suggest the development of Takotsubo cardiomyopathy. Due to the severity of the presentation of an acute Takotsubo event, the standard of care is to follow STEMI protocols for management (10). To date, there is no non-invasive test that can confirm Takotsubo cardiomyopathy. The gold standard is coronary angiography to rule out acute coronary syndrome (ACS) and thus suggest stress cardiomyopathy as a cause for the ST-elevation (11). Stress cardiomyopathy is otherwise managed symptomatically, focusing on aggressive treatment on any subsequent complications such as pulmonary edema or heart failure. Other complications of Takotsubo can include rhythm abnormalities such as atrial fibrillation, ventricular fibrillation, atrioventricular block, and cardiac arrest (6).
As Takotsubo cardiomyopathy and ACS may be clinically indeterminate, Takotsubo must remain a diagnosis of exclusion once ACS is ruled out with coronary angiography. Based on the patient’s presentation, the standard of care remains to treat any ST-elevation chest pain presentation is as a STEMI with subsequent cardiology consult for catheterization. Meanwhile, any significantly elevated troponin levels with associated chest pain should be treated as NSTEMI until further inpatient testing can be obtained. Nonetheless, it is important to keep stress myopathies on the differential when managing patients with presentations similar to what we demonstrate above.
Works Cited
- Daroff RB, Bradley WG, Jankovic J. Disorders of the Autonomic Nervous System. In: Bradley and Daroff’s Neurology in Clinical Practice. 8th ed. London: Elsevier; 2022:1930-1957.
- Olson PC., Agarwal V., Lafferty JC., Bekheit S.Takotsubo Cardiomyopathy precipitated by opiate withdrawal. H&L. 2017 November 2; 47: 73-75.
- Tutor A., Unis G., Ruiz B., Bolaji OA., Bob-Manuel T. Spectrum of Suspected Cardiomyopathy Due to COVID-19: A Case Series. Current Problems in Cardiology. 2021; 00.
- Daftari D., Savoj J., Skaf G., Gelman S., Aytyanont N., Heineke H., Biswas M. Who is at risk for cardiomyopathy in COVID 19. 2021 May 11; 77(18): 3034.
- Lang JP, Wang X, Moura F, et al. (2020). A current review of COVID-19 for the cardiovascular specialist. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7252118/pdf/main.pdf.
- Templin C et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015 Sep 3; 373(10):929-38.
- Sattar Y., Siew K., Connerney M. Management of Takotsubo Syndrome: a comprehensive review. 2020;12:e6556. doi: 10.7759/cureus.6556.
- Templin C., Ghadri J.R., Diekmann J. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938.
- Minhas A.S., Hughey A.B., Kolias T.J. Nationwide trends in reported incidence of takotsubo cardiomyopathy from 2006 to 2012. Am J Cardiol. 2015;116:1128–1131.
- De Giorgi A., Fabbian F., Pala M. Takotsubo cardiomyopathy and acute infectious diseases: a mini-review of case reports. 2015;66:257–261.
- Kasper D., Fauci A., Hauser S., Longo D., Jameson L., Loscalzo J. Harrison’s Principles of Internal Medicine 19th Ed. McGraw-Hill. 2015.